Low and Middle-Income Countries(LMIC’s) can and must play a leading role in determining the future of the world’s most advanced health technologies.
Gene therapy is at the forefront of modern medicine. By making precise changes to the human genome, these advanced technologies could lead to once-in-a-lifetime cures for infectious and non-infectious diseases (e.g., HIV, sickle cell disease) that affect tens of millions of people worldwide of whom live in LMICs.
Too often, the benefits of advanced health technologies are limited to high-income countries (HICs), which can be the case with gene therapies.
The narrative that new health technologies are not suitable for LMICs is a longstanding reason why most of the world is closed off from the benefits of modern medicine. Without concerted efforts to increase gene therapy capacity in LMICs, the global health gap will continue to widen.
The gene therapy industry is in its infancy, but early clinical advances and significant funding have provided tremendous momentum. This is an ideal moment for LMICs to enter the global market and prioritize the needs of the communities with the most diseases.
We asked five LMIC clinical researchers, all authors of a recently published white paper, what ground-breaking innovations and policy changes need to happen for gene therapy to have a real impact on global health.
Perform R&D where target diseases are prevalent.
Dr. Cissy Kityo Mutu za, Director, Uganda Joint Center for Clinical Research
While gene therapy has the potential to treat or even cure life-limiting diseases and infections, its full impact will only the achieved if we provide it for t benefit of all people, preferably. rather than increasing health inequalities between and within countries.
An important first step to maximizing the global impact of gene therapies is to build research and development (R&D) capacity in LMICs. Current gene therapy research has largely excluded LMICs, instead focusing preclinical and clinical work on HICs. Gene therapy R&D must be performed in areas where the target diseases are common to ensure the safety and efficacy of these therapies in these populations.
The manufacturing technologies and health infrastructure that are the source of cost for gene therapy products in HICs must be replaced by innovative and simplified platforms and workflows that reduce costs and are functional and cost-effective in LMIC health systems.
In terms of policy and regulation, individual countries must establish gene therapy frameworks that enable R&D.
The construction of such frameworks is guided by the recommendations of the World Health Organization, which emphasize safety, efficiency, and ethics. A critical component of effective global health interventions is community outreach. Treatment acceptability is essential for future clinical trials, so it is important that researchers and clinicians understand the risks and benefits of gene therapies.
Communication and training activities should be accessible to a wide range of stakeholders. Gene therapy and gene editing technologies are complex, and it can be difficult for the public to understand their potential benefits or side effects. However, patient and public support is critical to the successful adoption of any new technology.

Leveraging Pandemic Infrastructure and Policy
Professor Johnny Mahlangu, University of the Witwatersand South Africa
The ongoing COVID-19 pandemic is accelerating the innovation, deployment and a therapies of molecular therapies such as mRNA vaccines.
As a result, there is increasing interest in the development of molecular interventions for many other diseases, such as gene therapies for genetic diseases.
Strategic use of the infrastructure being developed for molecular therapy is critical to the production, testing, and delivery of gene therapies to various environments.
Three critical areas of consideration are:
- Repurposing manufacturing infrastructure developed during the pandemic to produce gene therapies; this approach reduces costs and accelerates deployment by maximizing the use of existing facilities and manpower. Expand existing national molecular therapy policies rapidly developed during the COVID-19 pandemic to include gene therapies.
- In the absence of a gene therapy policy, governments should create rapid ways to efficiently evaluate and approve these advanced drugs.
- Educating patients, health care providers, and funders about the value and impact of treating lifelong genetic diseases with gene therapy. This training should include professionalizing healthcare providers to become familiar with the processes involved in gene therapy, including safe product handling, drug infusion, and postprocedural patient monitoring.
Localizing manufacturing to reduce costs.
Professor Vikram Mathews, Christian Medical College, Vellore. India
Gene therapy is poised to revolutionize medical care for various diseases. Gene therapy is hoped to be a one-time curative therapeutic intervention for diseases ranging from inherited hemoglobinopathies such as sickle cell anemia and thalassemia to acquired diseases such as HIV.
A major challenge limiting the availability of these life-saving therapies is their astronomical cost, which makes them unaffordable even in developed countries, where most gene therapies originate. Due to economic challenges, there is often a mismatch between the regions of the world involved in the development and clinical trials and the regions of the world with the highest prevalence of disease targets.
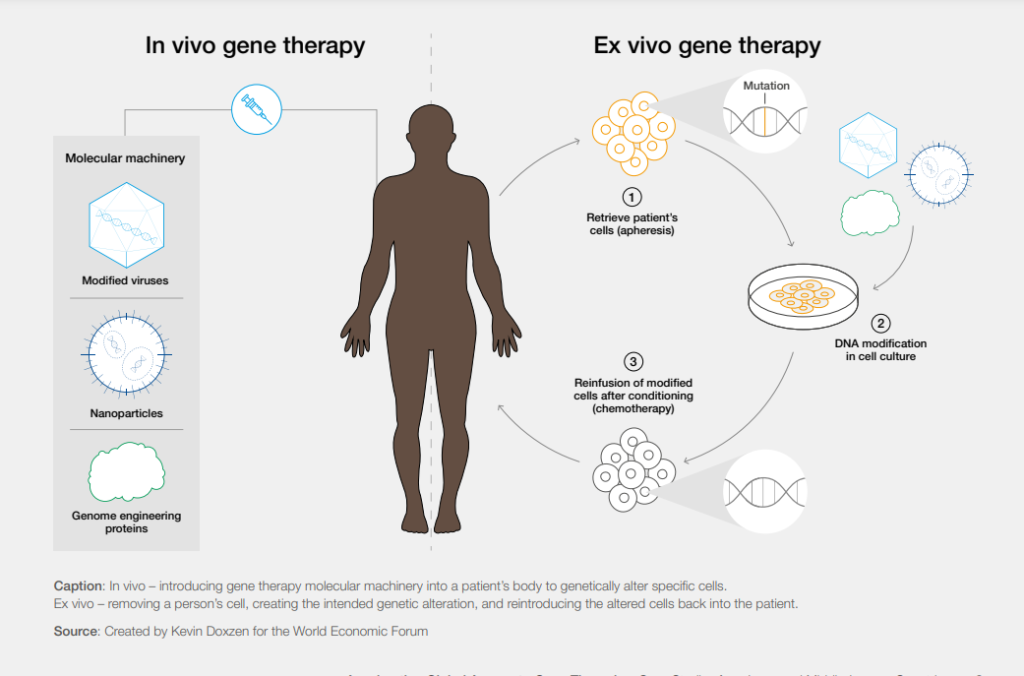
Classic examples of these are sickle cell anemia and HIV—the incidence of which is highest in Africa. Moving the production of gene therapy products to local areas and “points of care” (within hospitals) are strategies that can significantly reduce the cost and improve the availability of these products. In addition, current gene therapy methods use expensive ex vivo procedures that require removing the patient’s cells from the body. Instead, researchers must develop new in vivo methods that simplify the procedure with a single injection directly into the patient, saving time and money.
A multi-level approach to strengthening research
Professor Julie Makani, Muhimbili University of Health and Allied Sciences, Tanzania
For gene therapy to have an impact on global health, innovation and policy change must occur at multiple levels: individual, institutional, national, continental and global.
At the individual level, patients and staff are of primary importance. A patient-centered approach ensures that the community is involved in research and has a say in the adoption of a particular health intervention when available. In fields related to gene therapy, including health care, science, and education, it is necessary to increase the knowledge and opinions of employees about the progress and achievements of the field of gene therapy and to change their opinions.
At the national, continental, and global levels, genomic research is catalyzed by strategic partnerships and often takes place in centers of excellence (CoE). Many African countries have established centers of excellence in academic environments that combine health and research programs.
These innovative environments help maximize resources (physical and human) and create conditions that facilitate research and the translation of research results into concurrent health interventions in appropriate populations and geographies.
Investments in global health and gene therapy research must change at the political level. This can be done in three ways: direct investment in African institutions; raising the level of investment through financial partnerships; and recognizing that the duration of investments must be longer than the usual 3-5 year financing period.
Regulatory harmonization across countries
Professor Suradej Hongeng, Mahidol University, Thailand
Gene therapy has gained global attention in recent years, with recognition growing with each new clinical breakthrough. The industry is constantly evolving and innovations are disruptive in the public and private sectors. However, access to these life-saving treatments is limited by a number of technical and political challenges.
First, researchers must continue to develop cost-effective ways to deliver gene therapies to patients, an area of R&D where the private sector can play an important role. However, many LMICs have a weak ecosystem to support the creation of new businesses or to attract cooperation with international companies. Stronger involvement of the private sector is crucial to accessing emerging markets.
Second, the unique nature of these personalized therapies makes their regulation within traditional frameworks difficult, meaning that agencies must update existing policies and regulations. As the regulation evolves, it must align with the framework of other countries. This makes it easier for companies to navigate regulations and interact with agencies when conducting clinical trials or bringing a therapy to multiple markets.
You can read more about Global Gene Therapy Whitepaper here
Source:WEF




