James Kirkland began his career in 1982 as a geriatrician, treating aging patients. But he was not satisfied with what he could offer them.
“I got tired of prescribing wheelchairs, walkers, and incontinence devices,” recalls Kirkland, who now works at the Mayo Clinic in Rochester, Minnesota.
He knew that aging was considered the biggest risk factor for chronic disease, but he was frustrated by his inability to do anything about it. So Kirkland went back to school to gain anti-aging skills, earning a PhD in biochemistry from the University of Toronto. Today, he and his colleague Tamar Tchkonia, a Mayo Clinic molecular biologist, are leaders in a growing movement to halt chronic disease by protecting the brain and body from the biological consequences of aging.
If these researchers succeed, they won’t have a shortage of customers. People are living longer lives, and the number of Americans aged 65 and up will more than double to 80 million between 2000 and 2050.
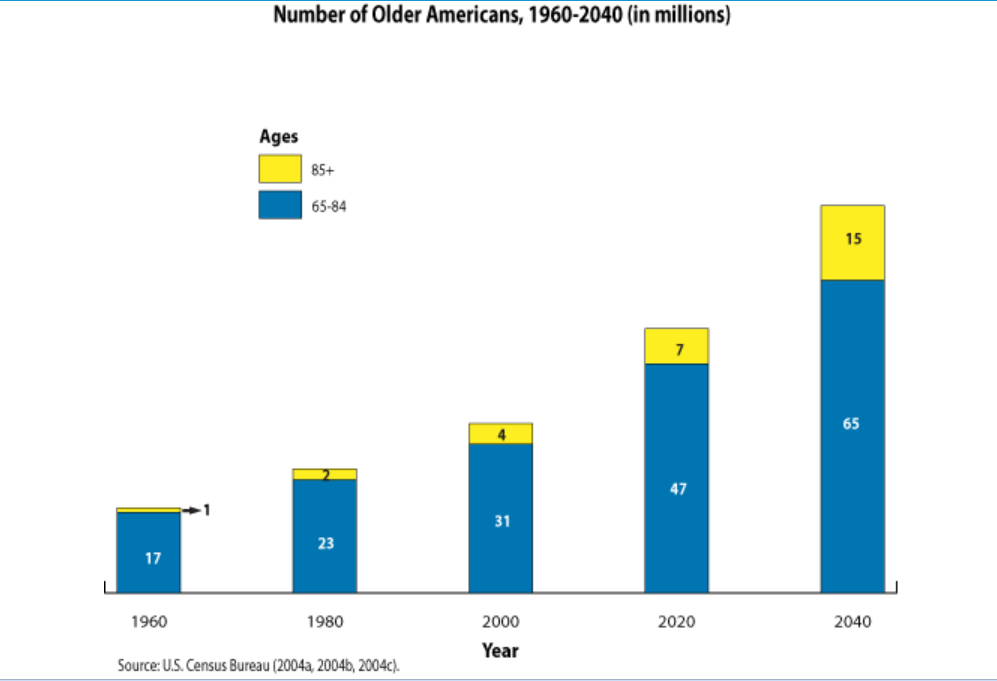
While researchers like Kirkland don’t expect life expectancy to increase, they do hope so. to extend the “healthy the length of time a person lives without disease.
One of their targets is degenerate cells that accumulate in tissues as people age. These “aging” cells have reached a point where they stop dividing but do not die, due to injury, stress, or simply time.
Although senescent cells normally make up only a small part of the total cell population, they made up up to 36 percent of the cells in some organs in aging mice, one study found. And they don’t just sit there quietly. Aging cells can release many compounds that create a toxic inflammatory environment that primes tissues for chronic disease. Senescent cells have been linked to diabetes, stroke, osteoporosis, and several other aging conditions.
These harmful cells and the idea that eliminating them can alleviate chronic diseases and the discomforts of aging are receiving serious attention. The US National Institutes of Health is investing 125 million dollars in a new research project called SenNet, which aims to identify and map aging cells in the human body and mice during their natural lifespans. The National Institute on Aging has awarded more than $3 million over four years to a multicenter Translational Geroscience Network (TGN) team led by Kirkland to conduct preliminary clinical trials of potential antiaging therapies.
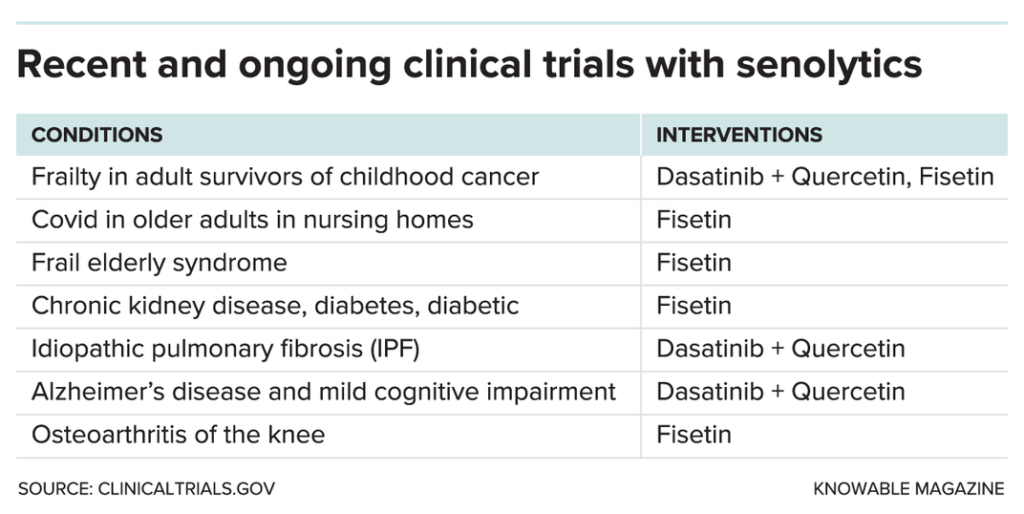
Drugs that kill aging cells, called senolytics, are among the best candidates. There are already small-scale studies of them in people with, for example, Alzheimer’s disease, osteoarthritis, and kidney disease.
“This is an emerging, incredibly exciting, and perhaps even game-changing field,” says John Varga, chief of rheumatology at the University of Michigan Medical School in Ann Arbor, who is not affiliated with TGN.
But he and others sound cautious, too, and some researchers think the field’s potential has been overstated. “There’s a lot going on,” says Varga. “I would say I have a very healthy skepticism.” He cautions his patients against many unknowns and says that trying a sebolytic supplement alone can be dangerous.
Scientists are still elucidating the biology of senescent cells, not only in aging animals but also in younger animals—even embryos, where the aging of certain cells is crucial for proper development. So far, the evidence that killing senescent cells improves health mostly comes from laboratory mice.
Only a few preliminary human trials have been conducted, with promising suggestions but far from breakthrough results.
However, Kirkland and Tchkonia hypothesize that senoliths may eventually help not only with aging but also with diseases caused by injuries or medical treatments such as chemotherapy in younger people. “Applications can be everywhere,” Kirkland muses.
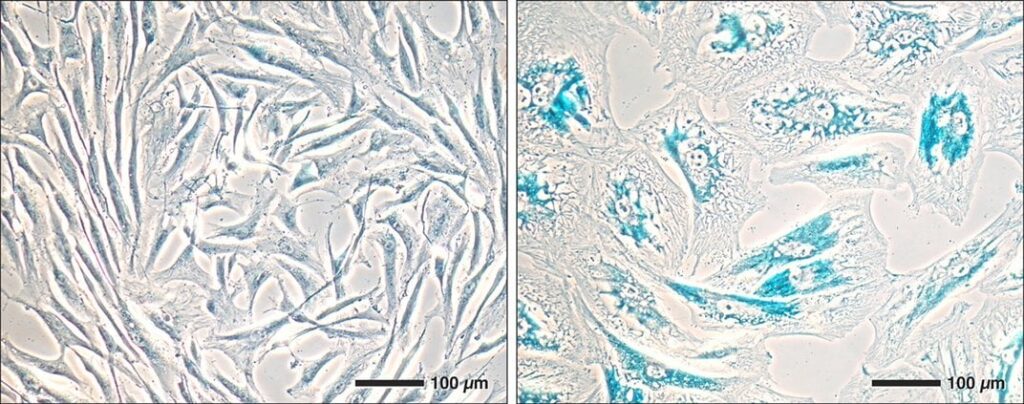
Good cells vs bad
Biologists first noticed aging when they began growing cells in laboratory dishes more than 60 years ago. After about 50 cycles, when cells first grew and then divided, the rate of cell division slowed down and finally stopped. When cells reach this senescent state, they grow larger and begin to exhibit various genetic abnormalities. They also collect extra lysosomes, sac-like organelles that break down cellular waste. Scientists have found a convenient way to detect many aging cells using stains that turn blue in the presence of the lysosomal enzyme beta-galactosidase, which is often overactive in these cells.
Scientists have also discovered hundreds of genes that senescent cells activate to shut down the cell’s reproduction cycle, changing their biology and suppressing natural self-destruction mechanisms. Some of these genes produce immune molecules, growth factors, and other compounds. The fact that certain genes are constantly turned on in aging cells suggests that there may be more to aging than just cellular depletion. This suggests that aging is a cellular program that evolved with a purpose in healthy bodies. Clues to this have come from studies conducted on creatures much earlier in their life cycle—even before birth.

Cell biologist Bill Keyes worked on embryonic aging in the early 2000s. When he stained whole mouse and chicken embryos with beta-galactosidase, small blue spots lit up in certain tissues. He soon met Manuel Serrano, a cell biologist at the Institute of Biomedical Research in Barcelona, who noticed the same thing. Cells bearing signs of aging appeared in the developing brain, ear and limbs, Keyes and Serrano reported in 2013.
Keyes, now at the Institute of Genetics and Molecular and Cell Biology in Strasbourg, France, focused on the limbs of mouse and chicken embryos. , where a strand of temporary tissue is formed at the ends of future toes. Unlike most embryonic cells, the cells of this coenid disappear before the animal is born.
They release chemicals that help the limb develop, and when their work is done, they die. At a molecular level, they look like old cells. Meanwhile, Serrano cells studied an organ that exists only in embryos: a temporary kidney called the mesonephric, which forms near the heart. When the definitive kidneys are formed, the mesonephric disappears. Here also beta-galactosidase and other aging-related compounds appeared in mouse embryos.
The cells in these temporary tissues are likely to disappear as they age. Certain compounds produced by aging cells call on the immune system and destroy the cells when their work is done. Scientists believe that the development of aging may be due to the short-term but crucial work of these cells.
Other studies show that senescent cells can also promote health in adult animals. Judith Campisi, a cell biologist at the Buck Institute for Aging Research in Novato, California, and others have found senescent cells in adult mice that are involved in wound healing. Connective tissue cells called fibroblasts fill the wound, but when they get stuck, they form abnormal scar tissue. During normal wound healing, they age and release compounds that promote tissue healing and invite immune cells to enter and destroy them.
In other words, the appearance of senescent cells in aging people is not necessarily a problem in itself. The problem seems to be that they take too long. Serrano suspects this happens because aging people’s immune systems can’t get rid of them all. And if senescent cells are left in place, the molecules they produce and the ongoing immune response can damage surrounding tissues.
Aging can also promote cancer, as described by Campisi in the Annual Review of Physiology, but the connection is multifaceted. Aging itself is an excellent defense against cancer – cells that do not divide do not form tumors. On the other hand, molecules released by aging cells can create an inflammatory, cancerous environment. So when a senescent cell gets close to a cell on its way to cancer, it can change the area enough to push a neighboring cell over the edge. In fact, Campisi reported in 2001 that injecting senescent cells into mice caused the tumors to grow faster.
Mice experiments
If aging cells in an aging body are bad, getting rid of them should be good. To test this idea, Mayo Clinic molecular cell biologist Darren Baker developed a way to kill senescent cells in mice. Baker genetically modified mice so that as their cells aged, they became sensitive to a certain drug. The researchers began injecting the drug twice a week once the mice turned 1 year old—that’s about middle age for a lab mouse.
Treated mice maintained healthier kidney, heart, muscle and fat tissue compared with untreated mice, and though they were still susceptible to cancer, tumors appeared later in life, the researchers reported in studies in 2011 and 2016. The rodents also lived, on average, five or six months longer.
These results generated plenty of interest, Baker recalls, and set senescence biology on the path toward clinical research. “That was the boom—a new era for cellular senescence,” says Viviana Perez, former program officer for the SenNet consortium at the National Institute on Aging.
Baker continued his research with mice that were genetically engineered to develop signs of Alzheimer’s disease. He said the removal of senescent cells prevented toxic proteins from building up in the brain and appeared to help the mice maintain their mental acuity, as measured by their ability to remember a new smell.
Of course, geriatricians cannot genetically engineer old people, so Kirkland, Tchkonia, and colleagues set out to find senolytic drugs that would kill senescent cells while leaving their healthy neighbors intact. They reasoned that because aging cells appear to be immune to a process called apoptosis, or programmed cell death, drugs that trigger this process may have senolytic properties.
Some cancer drugs do this, and the researchers included several of these in a screen of Six compounds were evaluated in vitro on senescent cells.The study turned up two major winners: one was the cancer drug dasatinib, an inhibitor of several natural enzymes that appears to make it possible for the senescent cells to self-destruct.
The other was quercetin, a natural antioxidant that’s responsible for the bitter flavor of apple peels and that also inhibits several cellular enzymes. Each drug worked best on senescent cells from different tissues, the scientists found, so they decided to use them both, in a combo called D Q, in studies with mice.
In one study, Tchkonia and Kirkland gave DQ to 20-month-old mice and found that the combination improved the rodents’ walking speed and endurance in lab tests, as well as their grip strength. The researchers reported that treating 2-year-old mice, the equivalent of 75- to 90-year-old humans, with DQ every other week extended their remaining lifespan by about 36 percent compared to mice that did not receive the senoliths in 2018. Tchkonia, Kirkland, and Baker hold a patent related to treating disease by eliminating senescent cells.
Clinical Trials
Scientists have since discovered several other drugs with anti-inflammatory effects, although DQ remains the favorite pair. Additional studies from several research groups have reported that senoliths protect mice against various aging conditions, including obesity-related metabolic disorders, atherosclerosis-related vascular problems, and osteoporosis-like bone loss.
“Collectively, this is a big deal,” says Laura Niedernhofer, a biochemist at the University of Minnesota Medical School in Minneapolis, a collaborator on some of these studies, and a member of the TGN Clinical Trials Collaborative. “It would be a shame not to test them on humans.”
Some small human trials have been completed.
The first, published in 2019, looked at idiopathic pulmonary fibrosis, a fatal condition in which the lungs become filled with thick scar tissue that impedes breathing. It is most common in people 60 and older, and there is no cure. In a small pilot study, Kirkland, Tchkonia, and colleagues administered DQ to 14people with the disease three times a week for three weeks.
They reported a significant improvement in participants’ ability to get up from a chair and walk for six minutes. But the study had significant caveats: In addition to its small size and short duration, there was no control group, and every participant knew they’d received DQ. Moreover, the patients’ lung function didn’t improve, nor did their frailty or overall health.
Niedernhofer, who wasn’t involved in the trial, calls the results a “soft landing”: There seemed to be something there, but no major benefits emerged. She says she would have been more impressed with the results if the treatment had reduced the scarring in the lungs.
The TGN is now running several small trials for conditions related to aging and other diseases, too. Kirkland believes that aging may be behind diseases that affect even the young, such as osteoarthritis from knee injuries and frailty in childhood cancer survivors.
Tchkonia and Kirkland, along with two companies, SpaceX and Axiom Space, are also studying how space radiation affects signs of aging in the blood and urine of astronauts.
They hypothesize that future long-duration journeys to Mars may require astronauts to monitor their bodies for aging or pack aging agents to prevent accelerated cellular aging caused by prolonged exposure to radiation.
Kirkland is also collaborating with researchers investigating the use of xenoliths to expand the range of available transplant organs. Despite the desperate need, about 24.
According to Stefan Tullius, director of transplant surgery at Brigham and Women’s Hospital in Boston,,000 organs from older donors are left out of the system each year because they are more likely to reject organs than younger donors. In heart transplant experiments with mice, he reported that pretreating older donor mice with DQ before transplanting them into younger recipients made the donor organs function “the same or slightly better” than hearts from young donors.
“It was huge,” says Tulio. He hopes to be able to conduct clinical trials in humans within three years.
Healthy skepticism
Many pharmaceutical companies are getting into antiaging, says Paul Robbins, a molecular biologist at the University of Minnesota School of Medicine. But the results were mixed. One pioneer, Unity Biotechnology of South San Francisco, California, withdrew from the pilot program in 2020 after its senolytic drug failed to reduce pain in knee osteoarthritis patients.
“I think we just don’t know enough about the right drug, the right delivery, the right patient, and the right biomarker,” says Varga of the University of Michigan, who is not affiliated with Unity. However, the company recently reported progress in slowing diabetic macular edema, a type of swelling in the back of the eye caused by high blood sugar.
Despite the excitement, Senolithic research is still in its infancy. Although the data from TGN’s initial small trials look good, they are not conclusive, says Robbins, a member of the network who believes positive results would be “a big deal.” The success of the small study suggests that it is worth investing in larger studies and developing drugs that are more effective or specific for aging cells.
“I urge extreme caution,” says Campisi, who is the founder of Unity and holds several patents for antiaging drugs. He is optimistic about the potential of aging research to improve health but fears that rushing senoliths into human trials, as some groups are doing, could set the field back. This is what happened to gene therapy in the late 1990s, when an experimental treatment killed a research volunteer. “I hope they’re serious about killing anybody,” he says.
Side effects are a constant concern. For example, dasatinib (D in Q) has many side effects, ranging from nosebleeds to fainting to paralysis.
But Kirkland thinks this may not be an insurmountable problem. He notes that these side effects only occur in cancer patients who take the drug regularly for months at a time, while anti-aging drugs may not need to be taken as often; once every two or three months may be enough to keep the population healthy and aging cells under control.
Another way to reduce the risks would be to make drugs that target aging cells in certain tissues, as Niedernhofer and Robbins note in the Annual Review of Pharmacology and Toxicology. For example, if a person has senescent cells in their heart, they can take a drug that targets only those cells and leave all the other senescent cells in the body, which can still do something good, alone.
For this strategy to work, doctors would need better ways to map aging cells in living people. While identifying such biomarkers is SenNet’s main goal, Campisi suspects that good ones will be hard to find. “It’s not a simple problem,” he says.
First, a lot of basic and clinical research needs to be done, but if all goes well, senolytics could one day be part of a personalized treatment plan: the right drugs at the right time to help keep the aging body healthy and mobile. It may be a long shot, but many researchers believe that getting many patients off walkers and wheelchairs is worth it.
Source: Knowable magazine, US gov
Get more news and insights about Global Biotechnology Industry here




